H&A PharmaChem owns Bioencapsulation as its main technology, and by connecting it to various fields, it promotes research and development of stable and differentiated cosmetic raw materials. Since 70% of all employees are researchers, they specialize in R&D field. We plan to continue to develop innovative technologies to achieve the best harmony between nature and technology through diverse research.
Office Address(Factory Address)
48, Bucheon-ro 186beon-gil, Bucheon-si, Gyeonggi-do, Republic of Korea. 14558
TEL / FAX / WEB
(+82)32-671-1798 / (+82)32-674-1798 www.10-9.co.kr
BIOENCAPSULATION
INVISIBLE ENCAPSULATION
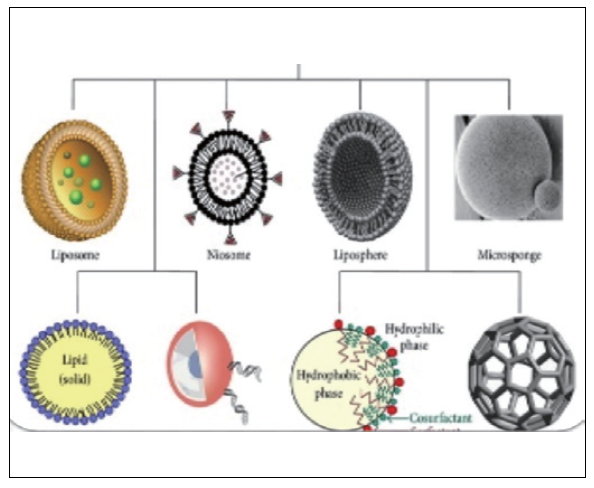
Transdermal Delivery Carrier Bioencapsulation technology can be used to dissolve active ingredients more effectively to maximize their efficacy, making it easy to apply to various cosmetic types.
Main Effect: Stabilization of raw materials, improved usability, improved skin per-meability, increased ease of use, and water-soluble substances
˙ Aqua Series˙Exosome˙Nanoceramide˙Cucurbituril˙Skin Lipid 334
˙ SLN, NLC, Niosome˙Vertisphere(Retinol)˙LNP, COCHELEATE˙+/- ion Liposome
VISIBLE ENCAPSULATION

Visible encapsulation stabilizes the active ingredients of cosmetics. Enhances visual effects, and performs charming and attractive ingredient to see and believe with eyes
Crystal Bead: Capsule's out appearance shines and can contain a various of functional oils inside - Microfluidics technology
Double Bead: Oil component has made as double layered structure General Sphere: After liposomize process, forming beads to minimize skin flexion
Large Bead: Cream has formed as a capsule, so it also known as cream bead Liquid Crystal Bead: Encapsulated L.C(Liquid Crystal) to form as Crystal Bead
˙Crystal Bead˙Double Bead˙General Sphere˙Large Bead˙Liquid Crystal Bead
IDEA PRODUCTS

We are developing innovative and creative products by further visualizing encapsulation technology
Drysome: Technique for minimizing the destruction of active ingredients by liposomeizing
Graphic Design: Filling technology that enables 3D characteristics (graphics) to be realized on cosmetics by applying robotics
Layered Double Hydroxide(LDH): As a new material technology, it can capture active ingredients of cosmetics between nanosheets and release them under certain conditions Metal-Organic-Framework(MOF/Poly-MOF): It is a structure of metal and organic matter. We have been conducting research and development to combine MOF with macromolecules to facilitate skin penetration with temperature and pH sensitivity. Recently, H&A have registered 10 INCI of Poly-LDH, Poly-MOF, 3D Biopringting
˙Drysome ˙Cleansing Ball˙Graphic Design˙Micro CRM˙LDH˙Aurora CRM˙MOF
˙Liquid CRM˙SUCRALFATE˙Retinal CRM˙Pearl Skin/CRM
<Patents> National: 46 / Overseas 10

◦LIPOSOME COMPOSITION FOR TREATING ACNE CONTAINING CONJUGATE OF LYSOPHOSPHATIDYLCHOLINE AND CHLORIN e6
◦ANTIBACTERIAL LIPOSOME COMPOSITION CONTAINING CONJUGATE OF PHEOPHORBIDE A AND LIPID DERIVATIVE OF POLYETHYLENE GLYCOL
◦NANO-EMULSION COMPOSITION OF COENZYME Q10
◦NANOEMULSION CONTAINING POLYASPARTIC ACID DERIVATIVE AND PERSONAL CARE COMPOSITION COMPRISING THE SAME
◦COMPOSITE FOR TRANSDERMAL TRANSFER USING METAL-ORGANIC FRAMEWORK AND NANOCELLULOSE
◦SILICONE GEL COMPOSITION AND METHOD FOR PREPARING GLOBULAR PARTICLE THEREFROM
◦COCHLEATES USING PHOSPHATIDYLSERINE/ANIONIC SURFACTANT/CALCIUM CHLORIDE
◦METHOD FOR PREPARING FREEZE-DRIED POWDER FACE PACK COMPRISING SEA SQUIRT EXTRACT
◦ORAL CARE COMPOSITION COMPRISING NANO-PARTICULATED CINNAMON EXTRACT
◦NANOSPHERE COMPRISING EGF, HARDY-ORANGE EXTRACTS AND TOCOPHEROL AND AN ORAL HYGIENE COMPOSITION CONTAINING THE NANO SPHERE
◦MULTILAMELLAR VESICLE CONTAINING RETINOL AND METHOD FOR PREPARING THEREOF
◦COMPOSITION FOR TRANSDERMAL DELIVERY COMPRISING NANOEMULSION AND MODIFIED LAYERED DOUBLE HYDROXIDE
◦METHOD FOR PREPARING MICRO FIBER USING NATURE-DERIVED POLYSACCHARIDE AND COSMETIC COMPOSITION COMPRISING MICRO FIBER PREPARED THEREFROM
◦NANO COMPOSITION FOR SOLUBILIZATION COMPRISINGAMPHOTERIC SURFACTANT AND POLYOL
History/Core Competency
2020~
2020.10 The 31st IFSCC congress 2020 Top 10 posters (Poly LDH)
2022.02 R&D Center re-located
2022. H&A won Award from Ministry of Trade, Industry and Energy
2018 ~ 2019
2018.12 Minister of Trade, Industry and Trade Award
2018, 2019, 2020 Youth-friendly steel enterprise
2014 ~ 2017
2017.03 CGMP Certified(KFDA)
2016.11 ISO22716 Certified
2015.03 Registration as Cosmetics manufacturer
2008 ~ 2013
2007.02 Patent acquisition (10-0687037)
2007.01 Company Laboratory Certified
2006.11 Patent acquisition (10-0645268)
2005.05 Patent acquisition (0489267)
2003 ~ 2007
2013.03 Conversion to a corporation
2013.02 Innovative VentureInno-biz Certified
2012.12 Company re-location
2011.09 ISO 9001 Certified
2002
2002.11 Establishment of H&A Pharmachem