ABSTRACT
Background: Antimelanogenic peptides are potentially useful to treat hyperpigmentation, but many peptides have limited application because of high cost and/or low activity.
Objectives: To identify small and potent peptide inhibitors of cellular melanin synthesis useful for cosmetic and medical applications.
Methods: A positional scanning synthetic tetrapeptide combinatorial library was used for screening of potentially active peptides. Antimelanogenic activities of the peptide pools and individual peptides were evaluated in B16-F10 melanoma cells and human epidermal melanocytes (HEMs) treated with alpha-melanocyte-stimulating hormone (α-MSH).
Results: The predicted active tetrapeptide sequences were R-(F/L)-(C/W)-(G/R)-NH2. Of the individual tetra-peptides tested, D3 (RFWG-NH2) and D5 (RLWG-NH2) exhibited high antimelanogenic activities. Tetrapeptide D9 (FRWG-NH2) with a sequence identical to that of a portion of α-MSH also showed antimelanogenic activity. Of the tripeptides tested, E5(FWG-NH2), E6(LWG-NH2), and E7(RWG-NH2) were relatively more active. Dipeptide F1 (WG-NH2) and monopeptide G1 (G-NH2, glycinamide) retained activity, but G2(Ac-G-NH2) and G3(glycine) did not. The antimelanogenic activities of peptides D3, E5, F1, and G1 were verified in α-MSH-stimulated HEMs. Commercially available G-NH2·HCl suppressed the phosphorylation levels of cAMP-responsive element binding protein, protein levels of microphthalmia-associated transcription factor and tyrosinase, L-tyrosine hydroxylase activity of tyrosinase, and the melanin levels in stimulated cells.
Conclusions: Small peptides, including glycinamide and tryptophanyl glycinamide, are potent antimelanogenic agents with potential value for the treatment of skin hyperpigmentation.
The team of pf. Boo, Yong-chool (Cell and matrix research institute, BK21 Plus, Department of Molecular Medicine, Kyungpook National University School of Medicine) have discovered a highly effective low molecular weight whitening peptide that can be applied to whitening functional cosmetics and treatment of skin pigment diseases.
About 80% of people are suffering from skin tone and pigmentation. And particularly in the case of melasma, age spot, and pigmentation after inflammation, they are not only a cosmetic aspect but also a medically important treatment target; however there are no safe and effective treatments yet. The pf. Boo’s team focused on the next-generation whitening peptide. Because peptides are composed of amino acids and biologically safe, and they can be expected to have various and unique effects depending on the amino acid sequence. The team of pf. Boo used PS-SCL screening techniques and predicted the optimized whitening peptide sequences among the possible 160,000 tetra-peptides. And finally they found a low molecular weight whitening peptide consisting of one to four amino acids. The action mechanism of these whitening peptides is to inhibit the binding of melanocyte stimulating hormone to the receptor and to block the signaling of the cell to inhibit the expression of melanin synthase.
Glycinamide, the whitening mono-peptide smallest in the world, is composed of one amino acid, and it is more than 20 times more effective than the conventional whitening agent arbutin in inhibiting cell melanin.
Professor Boo, Yong-chool said the significance of the research as follows: " The existing peptides were macromolecular substances which were expensive, unstable, and difficult to be absorbed into skin. While the newly discovered whitening peptide found by our research team was highly efficient low-molecular substance, so it is very useful in industrial and medical fields." Professor Boo has applied for a patent on whitening peptides with Ruby Crown, a venture company he founded, and is currently conducting clinical trials on human skin.
The study was performed with support from the 'Startup Growth Technology Development Project' of the Ministry of SMEs and StartupsMSS, and published in the online edition of British Journal of Dermatology on January 13, 2019.
Objectives
Human skin color is basically determined by genetic factors, but it also altered by acquired factors. Melanin pigment increases by skin aging, hormone secretion, ultraviolet ray exposure, inflammation reaction, and causes melasma, age spot, and pigmentation after inflammation
These skin pigmentation disorders are not fatal in themselves. However, since it can cause severe mental stress, it is being treated not only in terms of beauty but also in medicine. However, there is still no safe and effective treatment for skin pigment disease.
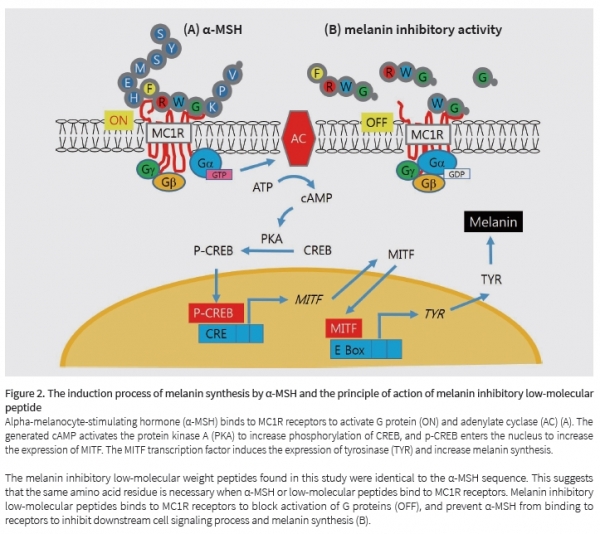
Interestingly, the alpha-melanocyte-stimulating hormoneα-MSH is a peptide consisting of 13 amino acids.
This hormone is secreted from the pituitary gland and skin, and binds to the MC1R receptor of melanocytes to initiate cell signaling. As a result, tyrosinase enzyme is expressed and melanin is synthesized. Our team has assumed that there would be peptides that prevent the binding of alpha-melanocyte-stimulating hormoneα-MSH to MC1R receptors. It was thought that if peptides first bound to MC1R receptors, α-MSH could not to bind to the receptors and cell signaling leading to melanin synthesis would be inhibited.
The aim of this study was to find low-molecular weight whitening peptides composed of less than 4 amino acids.
Method
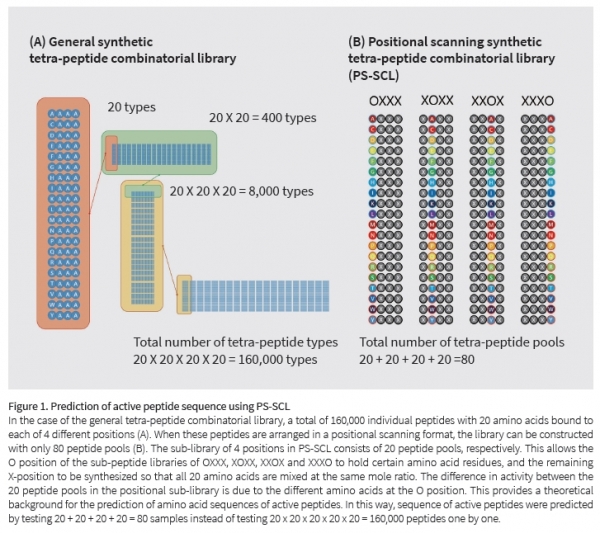
There are 20 kinds of amino acids that make up the peptide. Thus, there are 20 x 20 x 20 x 20 = 160,000 tetra-peptides with 4 amino acids connected. Since it is virtually impossible to experiment with all of these peptides, our team used positional scanning synthetic peptide combinatorial libraryPS-SCL. The PS-SCL tetra-peptide library consists of 20 + 20 + 20 + 20 = 80 pools. In the case of peptides in each grass, one position is determined by a specific amino acid. For the remaining 3 locations, 20 amino acids are mixed at the same ratio. Therefore, the activity of the peptide pool is determined by its specific amino acid residue.
With this method, the amino acid residues at positions 1, 2, 3 and 4 of the active peptide can be predicted.
Results
① When testing the tetrapeptide pool of PS-SCL in melanocytes stimulated by α-MSH, it was found that the Rarginine residue in position 1, the Fphenylalanine or Lleucine residue in position 2, the Ccysteine or Wtryptophan residue in position 3, and the Gglycine or Rarginine residue in position 4 played an important role in melanin inhibitory activity.
② Based on the results above, 8 tetra-peptides with R residue in position 1, F or L residue in position 2, C or W residue in position 3, and G or R residue in position 4 were synthesized individually. As a result, RFWG-NH2 and RLWG-NH2 had melanin inhibitory activity, while others were not. These active tetra-peptides were similar to α-MSH i.e. the FRWG sequences contained in Ac-SYSEMEHFRWGKPVNH2.
The additionally synthesized FRWG-NH2 also had melanin inhibitory activity.
③ Among tri-peptides in which several amino acids were excluded from active tetra-peptides, FWG-NH2, LWG-NH2 and RWG-NH2 had melanin inhibitory activity. Di-peptide WG-NH2 and monopeptide G-NH2 were also had melanin inhibitory activity, however Ac-G-NH2 and G had no activity.
④ G-NH2, the mono-peptide consisting of one amino acid, inhibited the phosphorylation of CREB induced by α-MSH, the expression of transcription factor MITF, and the expression of tyrosinase. G-NH2 (molecular weight 74) was much smaller than arbutin (molecular weight 272), however melanin inhibitory activity was 20 times more powerful.
Research implication
The first meaning of this study is the discovery of peptide having the strongest melanin inhibitory activity among the tetra-peptides through the screening method using the PS-SCL library. The second meaning is to find common point between the two sequences by comparing the sequences of these active peptides α-MSH. In other words, we have found the sequence of amino acids essential for binding to MC1R receptors.
The melanin inhibitory low molecular weight peptides found in this study act as MC1R antagonists. Since these low-molecular peptides are similar in structure to α-MSH hormones, they can bind to MC1R receptors in advance to prevent binding α-MSH hormones and receptors. In support of this, the low-molecular peptide inhibited phosphorylation of CREB induced by α-MSH, and also inhibited the expression of transcription factor MITF and tyrosinase. The third meaning of the study is that melanin inhibitory peptides found are highly effective low-molecular weight molecules. Since most existing peptides are polymers, they have various drawbacks such as high synthesis cost, difficulty in percutaneous absorption, and rapid biological degradation. In contrast, the peptides found in this study are not only low-molecular substance, but also have strong melanin inhibitory activity, so their industrial and medical applicability is very high.
In particular, G-NH2, a mono-peptide consisting of one amino acid, is the smallest whitening peptide in the world. Peptides are non-toxic biomaterials and have high biological activity, attracting attention as a next generation bio drug and cosmetics material. We are currently conducting clinical trials on the safety and efficacy of these peptides, and we expect breakthrough in the treatment of skin pigmentation.